Collecting Sample water
- The first phase in the boiler water testing is to collect feed water sample from the boiler concerned.
- Always take the water sample from the same place.
- Test samples usually cooled to 25 degree Celsius with the help of sample cooler. This is done to prevent flashing which concentrates the sample.
- If the sample is colored or turbid, filter paper should be used before testing. If the sample still remains turbid even after first filtration, the same filter paper should be used again as it will become more retentive after the second filtration.
- Allow the water to flow from sample cock before taking the sample for testing to make sure the line is clear of sediment
- The perfect location in the boiler to take sample is from the Salinometer Valve, after passing across a sample cooler making sure that the sample line is flushed through & sample bottle rinse completely.
- Allow the water over flow from the sample bottle to avert the air being trapped inside the bottle & keeping the bottle air tight for testing.
Alkalinity
Tests for alkalinity are as follows:
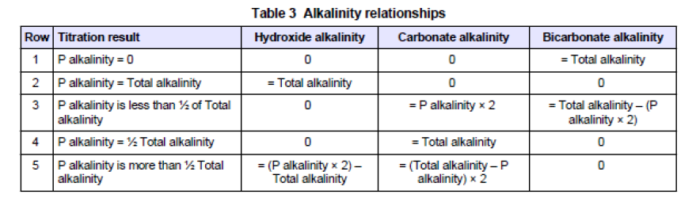
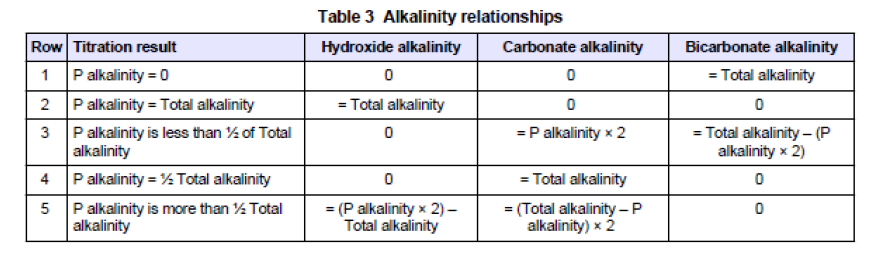
(1) Alkalinity to Phenolphthalein
Take 100 ml sample of boiler water,
add lml (10 drops) of Phenolphthalein,
add N/50 sulphuric acid to clear the sample.
Calculation: ml of N/50 acid used x 10 = p.p.m. CaC03
Phenolphthalein is less alkaline than hydroxides or carbonates, and when it is added to a sample containing hydroxides and or carbonates it will turn pink in color. The acid used after this coloration will first neutralize the hydroxides, forming salts, it will then react with the carbonate molecules present forming bicarbonate molecules. Bicarbonate molecules are less alkaline than phenolphthalein, hence, the pink coloration will vanish once all the hydroxides and carbonates have been dealt with by the acid. One bicarbonate molecule is formed from two carbonate molecules, hence in the test the quantity of acid used is a measure of the alkalinity due to the hydroxides (caustic) present and half the carbonates.
Ca(OH)2(aq)+H2SO4(aq)—→ CaSO4(s)+2H2O(l)
2CaCO3 + H2SO4 = Ca(HCO3)2 + CaSO4
Total Alkalinity
Take alkalinity to phenolphthalein sample,
add 10 drops of methyl-orange, result yellow coloration,
add N/50 sulphuric acid until pink
Calculation: ml of N/50 acid used for both tests x 10= p.p.m. CaC03.
Methyl-orange indicator is less alkaline than phenolphthalein and bicarbonates. It can be used initially in place of phenolphthalein or as is more usual, as a continuation after the alkalinity to phenolphthalein test. If no yellow coloration results when the methyl-orange is added to the alkalinity to phenolphthalein sample no bicarbonates are present. Hence no carbonates are present. Therefore, the alkalinity as determined in the alkalinity to phenolphthalein test has been due to hydroxides alone.
Note: Hydroxides & carbonates can co-exist together in a solution but hydroxides & bi-carbonates cannot.
Ca(HCO3)2 + H2SO4 → CaSO4 + 2H2O + 2CO2
H2SO4 + CaCO3 –> CaSO4 + H2O + CO2
Caustic Alkalinity
Take 100 ml sample of boiler water,
add 10 ml of barium chloride,
add 10 drops of phenolphthalein, result pink colouration,
add N/50 sulphuric acid to clear the sample.
Calculation: ml of N/50 acid used x 10 = p.p.m. CaC03
In this test barium chloride is added to the boiler water sample to precipitate all the carbonates which are present. The test is then carried out as for the alkalinity to phenolphthalein test but in this case only the hydroxides (caustic) will be measured.
BaCl2 + CaCO3 = BaCO3 + CaCl2
Ca(OH)2(aq)+H2SO4(aq)—→ CaSO4(s)+2H2O(l)
Chloride Test
Take alkalinity to phenolphthalein sample,
add 2 ml of sulphuric acid,
add 20 drops of potassium chromate indicator,
add N/35.5 silver nitrate solution till a brown colouration results.
Calculation: ml of N/35.5 solution used x 10 = p.p.m. CI
or ml of N/50 silver nitrate solution used x lO=p.p.m.CaC03.
Chlorides may be present in the boiler water sample & it is necessary that they be measured as they would be an indication of salt water leakage into the feed system, either a leaky condenser or a primed evaporator, etc. The alkalinity to phenolphthalein sample taken, has had the hydroxides and carbonates dealt with and they will play no further part in the test now conducted for chlorides. The sample is made definitely acidic by the addition of a further small quantity of acid, this is to speed up the chemical reactions which next take place. Silver nitrate has an affinity for potassium chromate and chlorides, its principal preference however is for the chlorides. When it has neutralized the chlorides present in the sample then it is free to react with the potassium chromate, in doing so it produces a reddish brown colouration. It is therefore apparent that the amount of silver nitrate solution used is a direct measure of the chloride content of the boiler water sample.
Note: As the drops of silver nitrate strike the sample, a reddish brown local colouration results which quickly disappears if chlorides are present. This should be ignored.
NaCl(aq) + AgNO3 (aq) = AgCl(s) + NaNO3(aq)
K2CrO4 + AgNO3 = Ag2CrO4 + KNO3
Sulphite Test
Take 100 ml of boiler water sample,
add 2 ml of sulphuric acid,
add 1 ml of starch solution
add potassium iodide-iodate solution till sample is blue in colour
Calculation: ml of iodide-iodate solution used x 12.5 =p.p.m. Na2S03.
The boiler water sample is made moderately acidic to speed up the chemical reactions which are to take place. Potassium iodide-iodate produces a blue colouration through reaction with starch, but it has a preferential chemical reaction with sulphite if it is present in the sample. Hence when the potassium iodide-iodate
solution has dealt with all the sulphite present, it is then free to react with the starch present in the sample, producing a blue colouration. It is therefore apparent that the amount of potassium iodide-iodate solution used is a direct measure of the sulphite content of the boiler water sample. As far as possible the atmosphere should be eliminated in this test otherwise an incorrect result may occur. If the test indicates that an sufficient reserve of sodium sulphite is present in the boiler water there is no need to conduct a test for dissolved oxygen.
4 KIO3 + 5 Na2SO3 + 7 H2SO4 = 2 I2 + 10 NaSO4 + 2 K2SO4 + 7 H2O
PHOSPHATE TEST
- Take 25 ml of the filtered boiler water sample
- Add 25 ml of vanodomolybdate reagent
- Fill comparator tube with this solution and place in right hand compartment of comparator
- In left had compartment place a blank prepared by mixing equal volumes of vanodomolybdate reagent & demonized water.
- Let the color to develop for at least 3 mins & then compare with disc.
- calculation: phosphate reserve in ppm(Parts per Million) from the disc reading