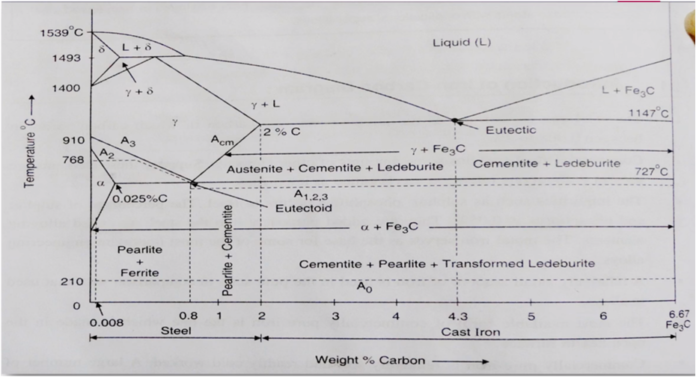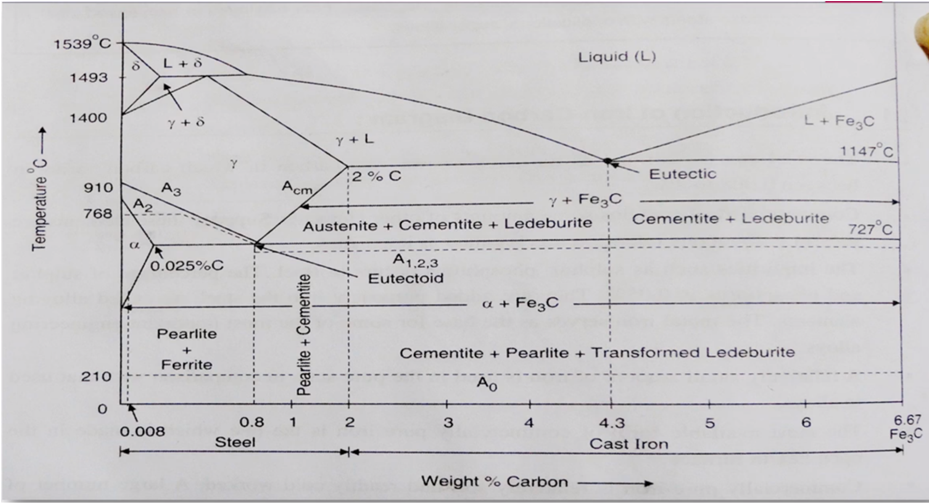
The part of iron and carbon alloy system line diagram representation of unalloyed iron and an interstitial compound, iron carbide (Fe3C), containing 6.67 percent carbon by weight is called iron-iron carbide equilibrium diagram. It should be noted that though it is called as equilibrium diagram, it is not a true equilibrium diagram, since equilibrium depends on no change of phase with time. In fact, the compound of iron carbide decomposes into iron and carbon. The decomposition takes a very long time at room temperature, even at 1300°F, it takes many years to form graphite. The iron carbide is called meta-stable phase. Therefore, iron-iron carbide diagram even though technically represents meta-stable conditions, can be considered as representing equilibrium changes, under conditions of comparatively slow heating and cooling.
DELTA FERRITE
- Solid solution of carbon in iron
- Soft and ductile phase, cold worked without cracking
- carbon is 0.09% at1493*C
- BCC structure
GAMMA AUSTENITE
- interstitial solid solution of carbon in Y-iron
- Solubility of carbon upto 2% at 1147*C
- Lowest temprature is 727*C at 0.8%C
- FCC structure
- Soft ductile malleable and non magnetic in phase
ALPHA FERRITE
- solid solution of carbon in a- iron
- Low solubility of carbon about 0.025% at 727*C
- BCC
- Soft and Ductile