Industrial Sources
The three major primary sources of NOx, in combustion processes:
Thermal Nox – Formed when nitrogen and oxygen in the combustion air supply combine at high flame temperatures. Thermal NOx is generally formed during the combustion of both gases and fuel oils.
Fuel Nox – Formed when nitrogen in the fuel combines with the excess oxygen in the combustion air and is only a problem with fuel oils containing fuel bound nitrogen.
Prompt NOx – Attributed to the reaction of atmospheric nitrogen, N2 with radicals such as C, CH, and CH, fragments derived from fuel Formed during the early, low temperature states of combustion and is insignificant.
Health Effects
NOx react with ammonia, moisture, and other compounds in atmosphere to form nitric acid vapor and related particles.
- Small particles can invade deeply into sensitive lung tissue and damage it, causing premature death in extreme cases
- Inhalation of such particles may cause or worsen respiratory diseases such as emphysema, bronchitis it may also aggravate existing heart disease.
- NOx (especially N,O) destroys ozone layer. This layer absorbs ultraviolet light, which is potentially damaging to life on earth.
- NOx react with common organic chemicals, and even ozone, to form a wide variety of toxic products: nitroarenes, nitrosamines and also the nitrate radical some of which may cause biological mutations.
Methods to Reduce/Control NOx production.
Primary
- Use of slide type Mini SAC or Zero SAC fuel valves
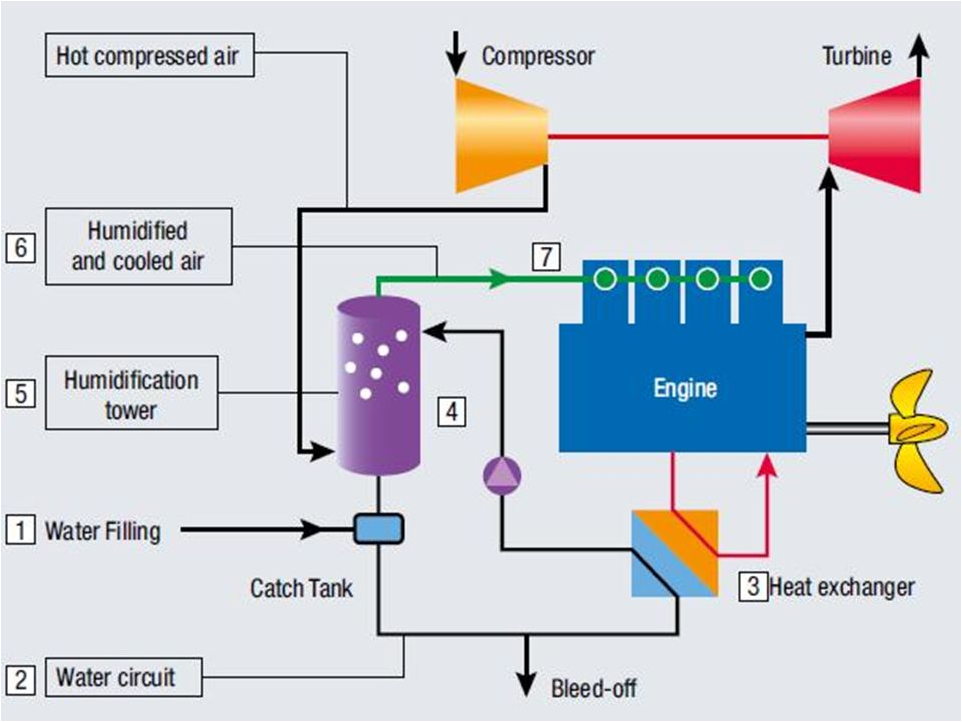
- Humid Air Motor (HAM) & (SAH)
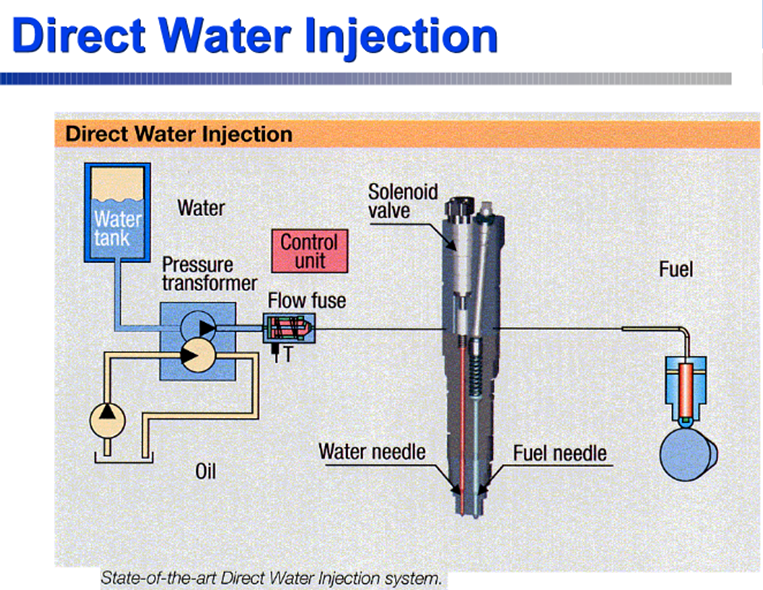
- Water injection with fuel
- Water emulsion with fuel
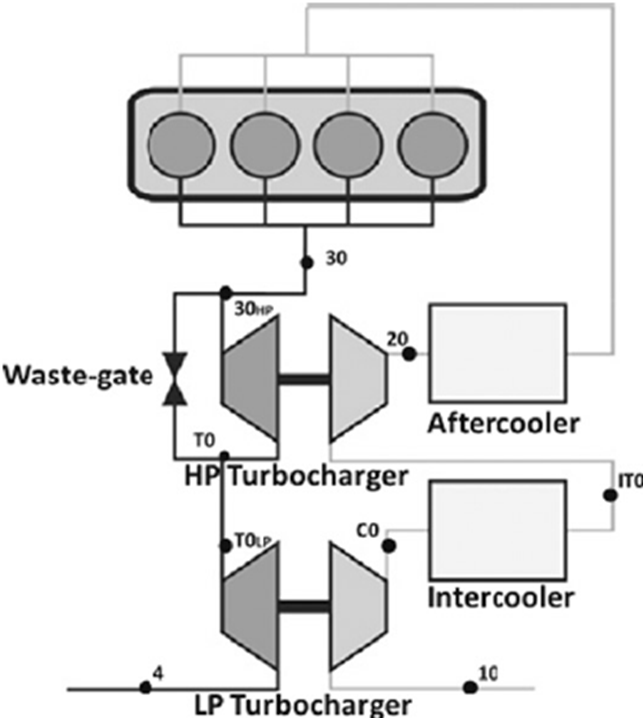
- Two stage turbo charger
- Millers cycle (4 stroke)
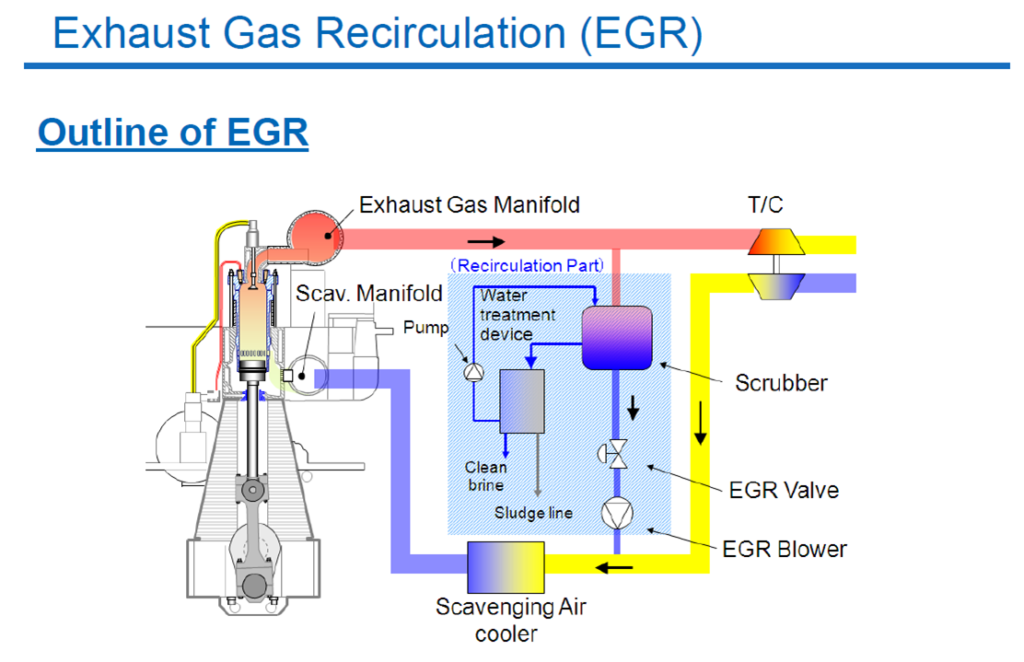
- Exhaust Gas Recirculation (ECR)
Secondary
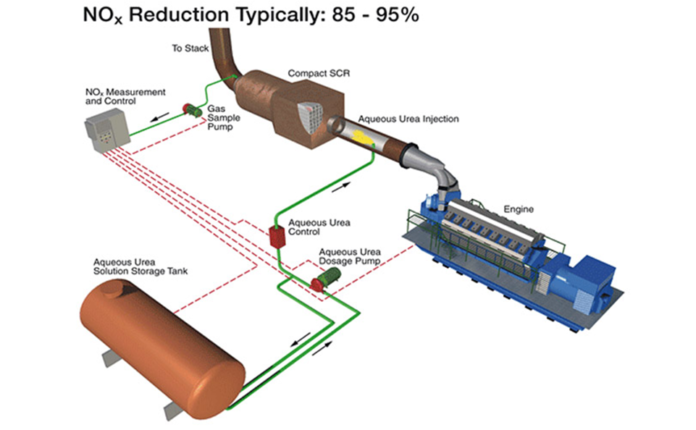
- SCR
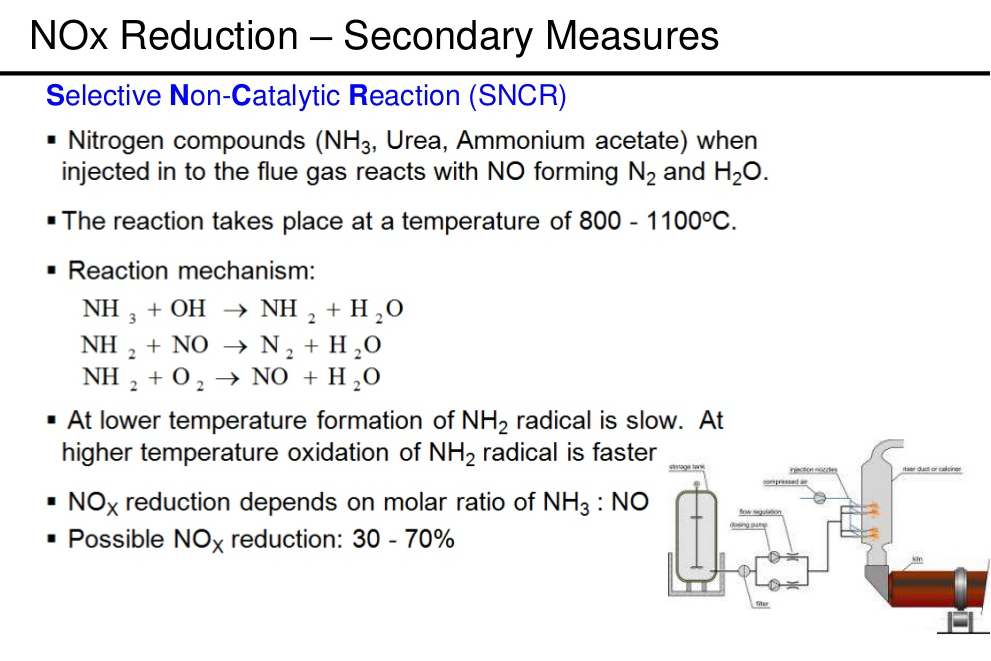
- SNCR
- CSNOx
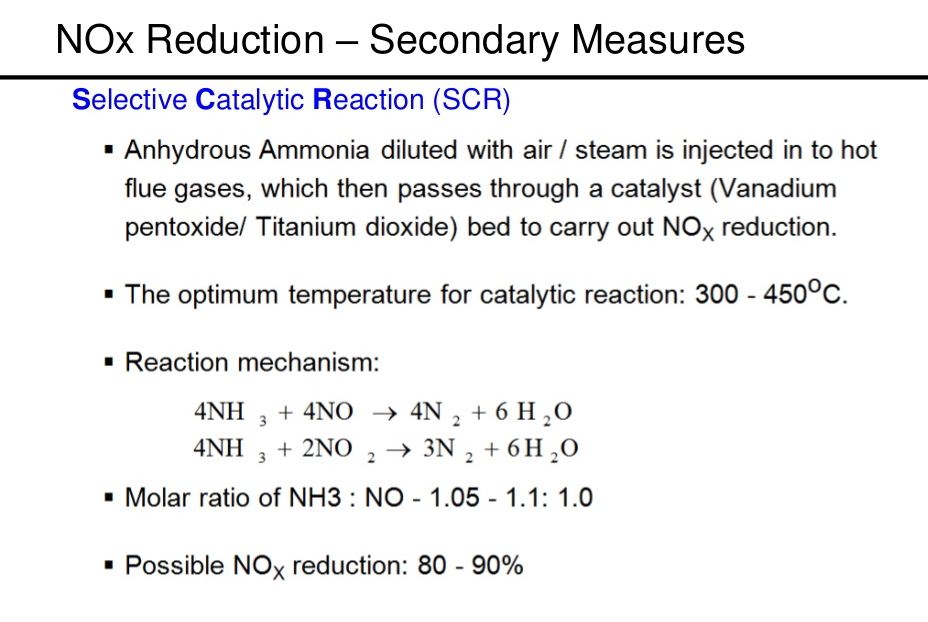
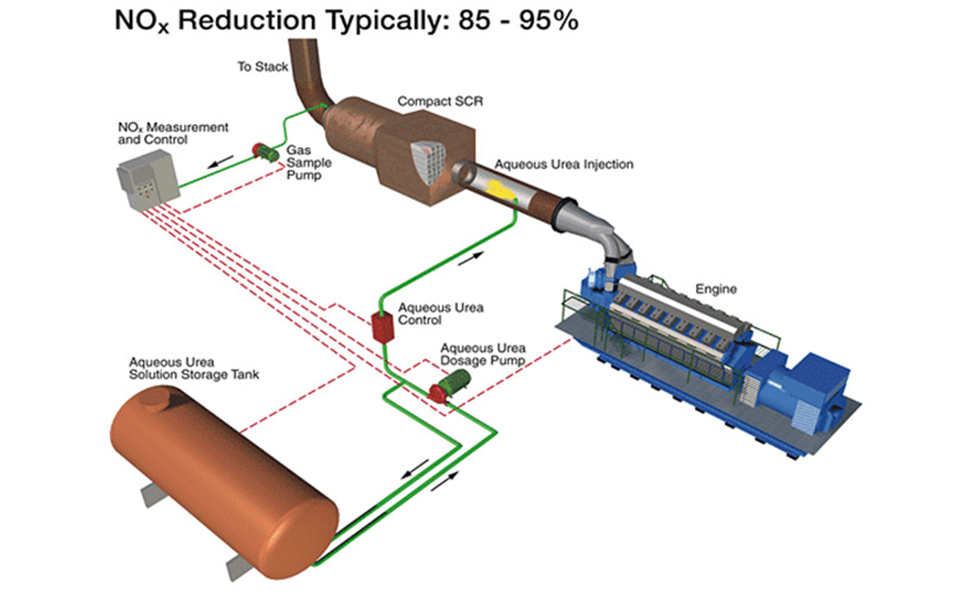
SCR – Limitation
- If materials are used in the manufacture of the units are unsuitable, ions can pass from the dispensing materials into the porous head on the SCR unit that spoils the SCR’s effectiveness and can reduce its lifespan by more than 60%.
- Ammonia slip or release of unreacted ammonia.
- Slip may occur when catalyst temperatures are not in the optimal range for the reaction or when too much ammonia is injected into the process.
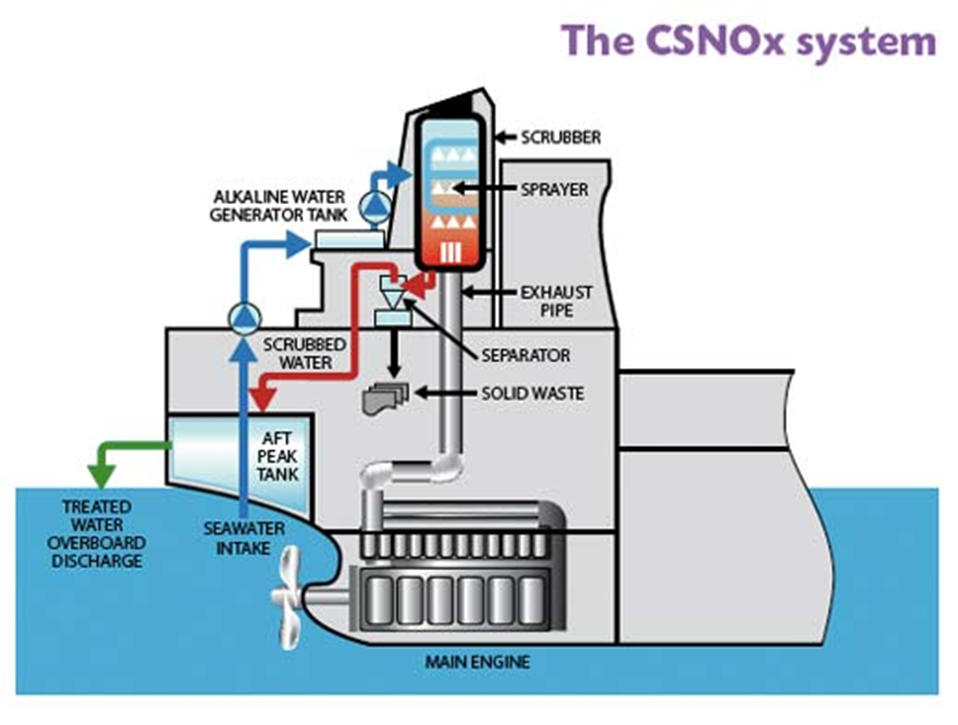
- In the CSNOx system, the pH value and alkalinity of scrub water is corrected prior to scrubbing using an ultra-low frequency electrolysis system (ULFELS).
- Seawater goes through an additional antifouling pre-treatment to control microbial growth in the system.
- The alkaline water is then pumped through the exhaust stack to scrub the flue gas.
- CSNOx treated water is highly reactive and effective in removing CO2, SO2 and NOx through absorption and that the removed pollutants are converted into the harmless substances found naturally in water.
- After scrubbing, the water which is scrubbed may pass through a solid-liquid separator to remove solid particles.
- The recovered water will then undergo an integrated treatment to meet the discharge water standard.
- If the water supply is under limit, the scrubbed water can be further treated and recycled back to the scrubbing process, reducing the amount used.