An electric battery is a device consists of two or more electrochemical cells that convert stored chemical energy into electrical energy. Each cell has +ve terminal (cathode) and a –ve terminal(anode). The terminal marked +ve is at a higher electrical potential energy than is the terminal marked-ve. The terminal marked +ve is the source of electrons that when connected to an external circuit will flow and deliver energy to an external device. Batteries are categorized into two groups.
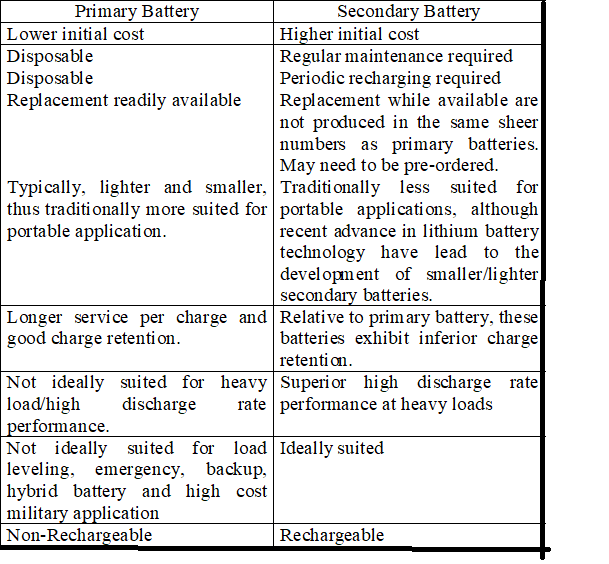
Primary Battery/Cell
Primary batteries are used one time and discarded, the electrode materials are irreversibly changed during discharge. In this the chemical action finally destroys one of the electrodes, usually the –ve. The primary cell can’t be recharged. The dry cell is the most popular type of primary battery.
It is ideal for simple application where an inexpensive and non critical source of electricity is all that is needed. The dry cell is not actually dry. The electrolyte is not in a liquid state, but is moist paste.
Common types of disposable batteries include Ionic-carbon batteries and Alkaline batteries.
Secondary Battery/Cell
Secondary can be discharged and recharged multiple times, the original composition of electrodes can be restored be reverse current. In this the chemical action alters the electrodes and electrolyte. The electrodes and electrolyte can be restored to their original condition by recharging the cell. Eg. Lead-acid batteries.
Lead Acid Battery
Each cell of a lead-acid battery contains two interleaved set of plates, immersed in electrolyte. These connected to the +ve terminal of a charged cell are of lead peroxide, those connected to the –ve terminal are of lead.The electrolyte in which the plates are immersed is a dilutes solution of sulphuric acid in distilled water.
Discharge action
During discharge, the hydrogen ions (H+) remove oxygen from the lead peroxide(PbO2) of the +ve plates and combine with it to form water (H2O).
Loss of oxygen from the lead peroxide shrink it to grey lead(Pb). The water formed by the action dilutes the electrolyte so that as the cell discharges, the specific gravity (relative density) decreases.
At the negative side of the cell, sulphate ions (SO– –) combine with the pure lead of the negative plates to form a layer of white lead sulphate (PbSO4). The lead sulphate layer increases during discharge and finally covers the active material of plate so that further reaction is stifled.
Positive Plate reaction:
PbO2 + HSO4 – + 3H+ + 2e– —-> PbSO4 +2H2O
Negative Plate reaction:
Pb +HSO4 – —> PbSO4 + H+ +2e –
Total reaction can be written as:
Pb +PbO2 + 2H2SO4 —> 2PbSO4 + 2H2O
Charging
To charge the cell is connected to a d.c. charging supply of the correct voltage. Flow of current from the charging source changes the discharge action of the cell: thus lead sulphate on the plates is broken down. The sulphate goes back into solution as sulphate ions(SO4 – –) , leaving the plate as pure lead.
Water in the electrolyte breaks down returning hydrogen ions(H+) to the solution, and allows the oxygen to recombine with the lead of the positive plate and form lead peroxide(PbO2).
Characteristics:
Fully Charged:
Specific gravity (Relative density) – 1.280
Voltage -1.95~2.2 V
Discharged:
Relative density – 1.120
Voltage – 1.8V
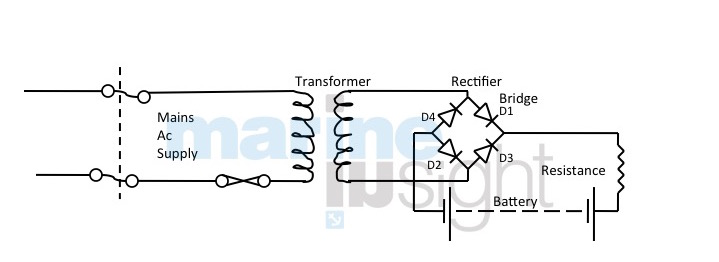
Battery Charging from A.C. mains
Main a.c. voltage is reduced by transformer to suitable value and then rectified to give a d.c. for charging. The supply current may be taken from the 230v section and charged to say 30v for charging 24v batteries.
Smoothing is not essential for battery charging but would be in cooperated for power supplies to low-pressure d.c. system with stand by batteries and for system with batteries on float.
Voltage is dropped in the transformer and then applied to the diodes which act as electrical non-return values. Each clockwise wave of current will travel to the batteries through D1 and returned through D2 (being blocked by other diodes). Each anticlockwise wave will pass through D3 and back through D4. Thus only current in one direction will reach the batteries.
Battery Room Inspection/Safeties
- Proper ventilation so that gases develop during charging escapes from battery room. Ventilation duct above accommodation level and should be aft of engine room blower.
- Ventilation provided by exhaust fan. The blades of exhaust fan should be of non-sparking material.
- Exhaust fan starting switch outside of battery room.
- Intrinsically safe instruments and tools to be used inside battery room.
- Lighting should be of explosion proof type.
- Distilled water container of PVC material. No steel container as it may react with distilled water. No glass as it can break as well as react with distilled water.
- Batteries placed on wooden blocks.
Inspection
- Battery installation and its charging rectifies.
- Battery room environment (Dry and well ventilated)
- Ventilation arrangement (as generates H2S gas)
- Battery tops clean and dry.
- Terminal nuts are tight (apply petroleum jelly prevent corrosion)
- Electrolyte level (maintain sp. Gravity, use hydrometer)
- Charging equipment (Dirt, over heating, loose connection)Avoid excessive rapid charging.
- No smoking and naked light allowed. (Notice)
- Vent holes should be kept clean. Batteries are secured properly.
- Flame proof light fittings are permitted.
- Ideal room temp – 15°C to 25°C (Battery life is shortened by temp rise above 50 and capacity is reduced by low temperature.
A hydrometer is a device that is used to measure the specific gravity(Relative Density) of liquids i.e. the ratio of the density of the liquid and the density of water.